What Are the Nonmetals in the Oxygen Family
In that location are 7 different elements classified as nonmetals: Hydrogen, Carbon, Nitrogen, Oxygen, Phosphorus, Sulfur, and Selenium.
The Halogens and Noble Gases are oft known as nonmetals too. Compared to metals, nonmetals are very poor conductors of electricity and rut. Nonmetals form acidic oxides, where every bit metals class basic oxides. The appearance of nonmetals (in solid form) is generally very dull. Nonmetals likewise tend to exist brittle; accept very depression densities, melting points, and boiling points.
Nonmetals make upward near of the earth's crust, atmosphere, and bodies of h2o. Living organisms are also composed of tissues, which are made upward of nonmetals. At room temperature, nonmetals exist in two of the three states of matter: gases and solids.
Hydrogen
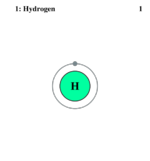
Electron Shell of Hydrogen
Hydrogen, not simply being the beginning element on the Periodic Table of Elements, is the nigh arable element in the whole universe. The word Hydrogen comes from the Greek words 'hydro'(water) and 'genes' (generate), thus explaining its many uses. The atomic symbol for Hydrogen is 'H'. It'due south diminutive number is 1, and it's diminutive mass is 1.00794 g/mol. Hydrogen is completely odorless, colorless, and tasteless. Information technology is known as the most flammable of all known substances, and about 75% of the most abundant compounds contain hydrogen. Hydrogen is the just element to have three isotopes that each have their own names. These isotopes are: Protium, Deuterium, and Tritium. Each of these isotopes have one proton, one electron to residuum it, and unlike numbers of neutrons. Protium has no neutrons, Deuterium has one neutron, and Tritium has two neutrons.
Hydrogen has the potential to be a very efficient fuel source. Hydrogen is virtually commonly obtained from mines, oil, and gas wells. 1 of the biggest uses of Hydrogen is a fuel for combustion. Combustion of Hydrogen works in internal combustion engines, gas turbines, and in rocket ships or jets.
Hydrogen is besides used in fuel cells. A fuel cell is a device that produces a continuous electrical current from the combination of hydrogen with oxygen, and/or some other substance. For instance, fuel cells are used on the infinite shuttle to provide electricity needed for the operation of the shuttle. The combination of Hydrogen, Oxygen, and other substances creates electricity, estrus, and breathable oxygen for the astronauts aboard. There are thousands of things Hydrogen is used for; it is used to produce: ammonia, gasoline, heating oil, fertilizer, glass, metallic, vitamins, makeup, soaps, and even margarine and peanut butter! Also, wind ability and hydroelectric plants produce Hydrogen in gild to store energy during off peak times.
Hydrogen was first recognized equally a singled-out substance in 1766 by Henry Cavendish, an English pharmacist and physicist. Hydrogen is found in bang-up abundance in many stars and planets. Hydrogen is most always found in its atomic and plasma states. About of the hydrogen establish on the Earth is found in the grade of chemic compounds, such as hydrocarbons and water.
Carbon


Carbon is the sixth chemical element on the periodic table. It's symbol on the Periodic Table is 'C', its atomic number is vi, and its atomic mass is 12.0107. It has six protons and six electorons.
Two of the most mutual forms of carbon are graphite and diamond. Even though they are made upward of the same element, they have unlike structures due to the bonding differences. Diamond is the hardest substance in the world and is a groovy electrical insulator. Graphite is one of the softest minerals on the world and is a good conductor of electricity. Diamond has a very potent and rigid structure because it is strongly covalently bonded. Graphite is very breakable. Even though it is covalently bonded, information technology is easily broken due to its weak intermolecular bonds.
Carbon has the ability to form covalent bonds with itself, creating concatenation or ring molecules. This ability is known as catenation. Catenation takes place in other elements, merely carbon is the well-nigh well-known chemical element for its power to practice then.
Carbon has 4 different allotropes: amorphous, diamond, graphite, and fullerenes. A 5th allotrope has recently been discovered that is very lightweight, spongy, and is attracted to magnets.
Nitrogen
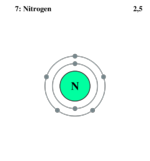
Electron Crush of Nitrogen

Nitrogen is the most mutual element in the globe'due south atmosphere, making up seventy% of present gases. Nitrogen is essential for living organisms and plays a large part in the amino acids and living tissues that make them up. Nitrogen is a steadily reacting element and more often than not reacts with elements, such as lithium and magnesium, to class nitrides. In the atmosphere, information technology is found every bit a gas or in stone layers. Nitrogen gas is colorless, odorless, and tasteless. Nitrogen is trivalent to most bonds because its atoms are unremarkably found in pairs every bit diatomic models, bonding with themselves.
Interestingly, Nitrogen is used in the formation of stars and occurs throughout outer space. The element itself is actually created by the Sunday and stars. The temper of Jupiter's moon, Titan, is made up about entirely of Nitrogen.
Being used for natural and artificial growth in plants, farmers rely on Nitrogen for the product of their crops. In the legume ingather, Nitrogen is used for restoring the soil, making it fertile for other crops to grow once more. Since Nitrogen is an inhert gas, it is also used as a tool for chemists, unremarkably as a shield to keep oxygen reacting with other elements. Also, known for its cooling abilities, it is oftentimes used in food freezers. Because it is then unreactive, it is sometimes used to fill lightbulbs.
Oxygen


Oxygen, being the near widely known element in the world, is the 3rd most abundant element in the dominicus and is used in the earth'due south carbon-nitrogen bike. Oxygen is very reactive, and is a major component in thousands and thousands of compunds. It will form oxides with all of the elements on the Periodic Tabular array, except for Helium(He), Neon(Ne), Argon(Ar), and Krypton(Kr). Information technology is normally in air class, and when turned into a liquid or a solid, information technology is a clear or light blue colour. Oxygen is odorless and tasteless, is slightly heavier than air, and is a little scrap soluble in water.
Oxygen is the biggest component of the world's crust, and its second biggest occurence is in the Earth's atmosphere. Oxygen is unremarkably found in the form of silicates or water. Silicates are an oxide of silicon. Oxygen makes upward two-thirds of water's (H2O) weight.
Oxygen has thousands of uses. Probably Oxygen's almost obvious use is in the Earth'due south atmosphere, because we demand information technology to breathe and stay alive. Oxygen is also a necessity for creating fire. Plants and animals could not survive without oxygen, because, just like humans, they need respiration. Oxygen is often used in hospitals to treat patients with pneumonia, gas poisoning, and other ailments. Information technology is very important in iron and steel industries. Oxygen is also used as a liquid-class oxidizer in fuel systems of rockets.
Phosphorus

Electron Crush of Phosphorus

Phosphorus, existence an extremely reactive element, cannot exist found as a complimentary chemical element in nature. It is in the nitrogen grouping and is a multivalent nonmetal. Phosphorus, being transparent and colorless in pure form, is mostly a waxy, white solid. Phosphorus is insoluble in water, however soluble in carbon disulfide. Information technology is highly poisonous considering information technology burns in air to its penoxide, and can cause severe burns when it comes in contact with peel.
Phosphorus has four forms of allotropes, which are 2 forms of white, red, and black. White phosphorus is converted to cerise phosphorus when it is heated or exposed to sunlight. Red phosphorus can actually vary in color, from orange to royal. Cherry-red phosphorus, different white phosphorus, does non phosphoresce in air. Black phosphorus has the ability to conduct electricity, as it is fabricated under loftier force per unit area and looks and feels like graphite.
Phosphorus can exist constitute in many different minerals. Phosphate rocks are almost abundantly found in Mainland china, Russia, Morocco, Florida, Idaho, Tennessee, and Utah. Phosphorus has thousands of uses, such equally: smoke bombs, condom matches, pyrotechnics, incendiary shells, tracer bullets, fireworks, pesticides, toothpaste, shampoos, detergents, explosives, and nerve agents.
Sulfur


Sulfur is a non-metal and a multivalent element, and can be found most everywhere on the earth. It has a very singled-out odor, often described as that of a rotten egg. When Sulfur is burned, it burns with a blueish flame and releases Sulfur Dioxide. Sulfur can form compounds with all the elements on the periodic table except for the Noble Gases. Sulfur is insoluble in h2o, however soluble in Carbon Disulfide. Sulfur is also known for its crystallography. The allotrpes of sulfur form rhombic and monoclinic crystal structures. At room temperature, an baggy form of Sulfur is metestable and so begins to course into crystalline. This transformation process usually takes several hours, or even days, but on rare occasions, takes just a few minutes.
Sulfur is almost abundantly found about hot springs and volcanoes. The Pacific Ring of Fire, a large ring of volcanoes, has many hot springs and volcanoes with an abundance of sulfur. Sulfur deposits are believed to be originated from the action of anaerobic bacteria on sulfate minerals.
The metal sulfides similar pyrite, which is iron sulfide, cinnabar, which is mercury sulfide, galena, which is lead sulfide, sphalerite, which is zinc sulfide, stibnite, which is antimony sulfide are categorized as common naturally occurring sulfur compounds. The metal sulfates similar gypsum, which is calcium sulfate, alunite, which is potassium aluminium sulfate, and barite, which is barium sulfate are also categorized as common naturally occurring sulfur compounds.
Selenium
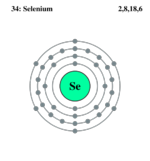
Electron Beat of Selenium

Selenium only occurs rarely in a free state of nature. Beingness a nonmetal, it is chemically similiar to Sulfur and Tellurium. Selenium occurs in several different forms, but the nigh stable of these is a dense purplish-gray form that has the structure of a trigonal polymer concatenation. It is toxic in abundant amounts, but small amounts of it are necessary for the function of cells in all animals.
Selenium conducts electricity very well in the night and is used in photocells. It also exists in many nonconductive forms, such equally: a black glassy substance, and red crystalline forms built of ring molecules with eight members.
References
- Nonmetals Wikipedia
- Periodic Tabular array: Nonmetals ChemicalElements.com
- Metals vs. Nonmetals Tod Shipman
- Properties of the Nonmetals All Info Virtually Chemistry
- Selenium Wikipedia
| Chemistry | ||
|---|---|---|
| Featured articles | Atom • Biotechnology • Chemical bonds • Chemical series • Substances • Compounds • Chromatography • Electrons • Energy • Elements • History of the Periodic Table • Ion • Microscopy • Nuclear chemistry • Nuclear weapon • Organic chemical compound • Periodic tabular array • Radioactivity • State of matter |  |
| Compounds | Acid • Booze • Amine • Amino acrid • Anion • Base • Sugar • Chloride • Carboxylic acid • Electrolyte • Hydrocarbon • Hydroxide • Inorganic chemical compound • Ion • Isomer • Lipid • Nucleotide • Nitrate • Organic • Phenol • Polyatomic ion • Protein • Resonance structure • Salt • Sugar • Sulfate • Sulfide | |
| | ||
| Periodic Table of Elements | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Exist | B | C | Northward | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| Grand | Ca | Sc | Ti | V | Cr | Mn | Atomic number 26 | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Atomic number 82 | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
| |||||||||||||||||||||||||||||||||||||||||
Source: https://www.creationwiki.org/Nonmetal
0 Response to "What Are the Nonmetals in the Oxygen Family"
Post a Comment